Modulated Charge iHILIC® columns
-
Analytical (2.1mm - 4.6mmID) iHILIC® Fusion and Fusion(+) columns used in an ERLIC vs. HILIC mode.
Column Publications
Applications
Part Numbers & Prices:
-
Fusion 1.8µm UPLC & 3.5µm, 100Å columns & guards (slightly negative surface)
Fusion (+) 3.5µm, 100Å columns & guards (slightly positive surface)
Fusion (P) 5µm, 200Å columns (for high pH metabolomics, inorganic ions and nTP's)
iHILIC®-(P) Classic 5µm, 200Å columns (for metabolomics, inorganic ions and nTP methods developed on ZIC® pHILIC columns). Operating Instructions/Chemistry
iSPE Publications
Addenda:
pH Range of Buffers Suitable for LC-MS Operation
"Addressing a Common Misconception: Ammonium Acetate as Neutral pH "Buffer" for Native Electrospray Mass Spectrometry." Konermann, L., J Am Soc Mass Spectrom. 2017 Sep;28(9):1827-1835. doi: 10.1007/s13361-017-1739-3. Epub 2017 Jul 14.

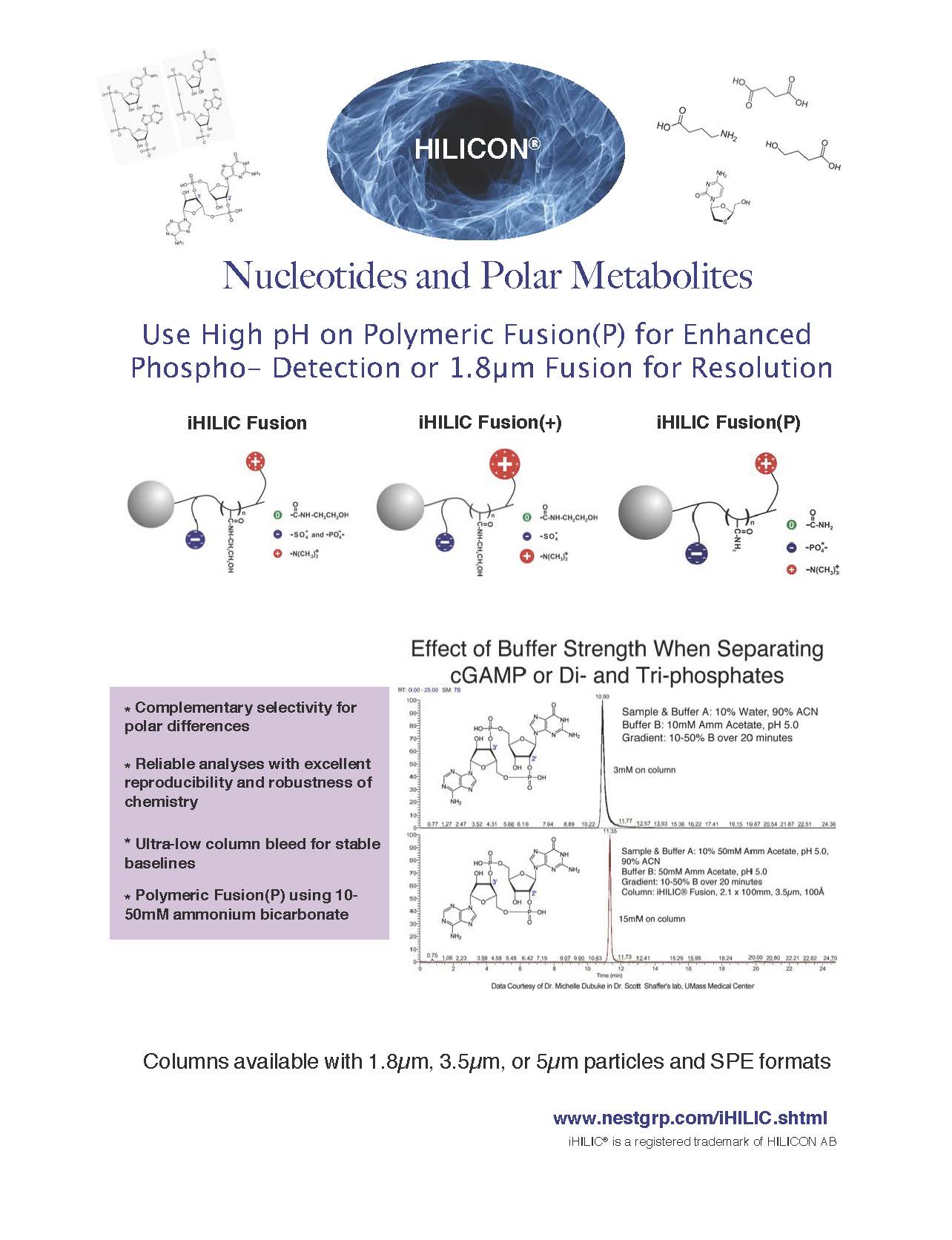
Effect of Buffer Strength on iHILIC® columns


Menu
Publications:
- iHILIC® Column Publications
iSPE® Publications
LCGC Application Notes
Posters
Application Library by Chemical Type
Applications:
- Amino Acids
![]()
![]()
Gradient elution: A) acetonitrile; B) 10mM ammonium formate,
pH = 3.5; gradient elution from (95/5) A/B to (84/16) A/B in 8.5 min.
Column temperature: 40 °C
Injection volume: 5 µL
Data Courtesy of University of Münster
Mono- and di-saccharides
![]()
Isocratic 80:20 ACN 25mM ammonium acetate (v/v)
Column temperature: 35 °C
Injection volume: 10 µL
Data Courtesy of Exact Scientific Services
Glucuronides and Glucosides
![]()
Gradient 90:10 to 30:70; ACN:10mM, pH 3.4, ammonium acetate w/0.1% FA (v/v)), 10µL injection.
Menu
UDP-Glucose
![]()
![]()
Gradient 80:20 to 60:40; ACN:100mM, pH 5.8, ammonium acetate (v/v))
Publication: "Metabolic chemical reporters of glycans exhibit cell-type selective metabolism and glycoprotein labeling." Anna R. Bhatt, et al., ChemBioChem 10.1002/cbic.201700020
cGAMP
![]()
Gradient 90:10 to 50:50; ACN:50mM, pH 5, ammonium acetate (v/v))
Menu
Mono-, Di- & Tri-phosphate Nucleotides
![]()
Nucleotides Separate at a lower buffer concentration on iHILIC® Fusion at pH 5.8, 70:30; ACN:100mM, pH 5.8, ammonium acetate (v/v) than at pH 4.5, 70:30; ACN:100mM, on ZIC® cHILIC due to less titrating to lower the pH.
Enhanced sensitivity of phosphates by MS at high pH:
![]()
iHILIC® Fusion(P), 4.6 x 150mm, 200Å, 5µm.
Separation of nTP's at pH 9.3 with a volatile buffer: Gradient. Buffer A: 80:20 (v/v) Acetonitrile, 100mM ammonium bicarbonate and Buffer B: 30mM ammonium bicarbonate pH 9.3.
Metabolomics (ATP/ADP ratios, acetyl-CoA). "Breast Cancer-Derived Lung Metastases Show Increased Pyruvate
Carboxylase-Dependent Anaplerosis," Christen et al., 2016, Cell Reports 17, 837–848
October 11, 2016 and supplemental information. MS in negative mode. Column iHilic Fusion(P). Solvent: ACN and pH=9.3, 10mM ammonium acetate.
Separation of 2'NADP from 3'NADP at high pH:
![]()
![]()
iHILIC® Fusion(P), 4.6 x 150mm, 200Å, 5µm.
Separation of NADP's at pH 9.3 with a volatile buffer: Gradient. Buffer A: 80:20 (v/v) Acetonitrile, 20mM ammonium bicarbonate and Buffer B: 20mM ammonium bicarbonate pH 9.3.
Unpublished data courtesy of Corey D. Broeckling, Colorado State University.
Menu
Organic Acids:
![]()
![]()
![]()
iHILIC® Fusion(P) PEEK, 2.1 x 50mm, 5µm, 200Å
Isocratic separation of γ-hydroxybutyric acid (GHB), γ-aminobutyric acid (GABA) & succinic acid with a volatile buffer: Buffer A: 90:10 (v/v) Acetonitrile, 20mM ammonium acetate.
Unpublished data courtesy of Patrick Belanger, INSPQ - Centre de Toxicologie (CTQ). See also: Effects of pH and Buffer Strength, an Organic Acids Study.
Phospholipids HPLC:
![]()
![]()
iHILIC® Fusion, 2.1 x 150mm, 3.5µm, 100Å.
* Double peak of PA is caused by
in-source fragmentation of the PS head group resulting in
corresponding PA species.
Journal of Chromatography A, 1565 (2018) 105–113
Eluent: A) Ammonium acetate solution (35 mM, pH 5.75)
and acetonitrile (95:5, v/v); B) Acetonitrile.
Gradient elution: 0-0.5 min, 97% B; 0.5-26.5 min, from 97%
to 75% B; 27-33 min, 60% B; 35-45 min 97% B.
Flow rate: 0.3 mL/min
Column temperature: 40 °C, Injection volume: 10 μL
Phospholipid standards: Bis(monoacylglycero)phosphate
(BMP 36:2), phosphatedylglycerol (PG 36:2),
phosphatidylcholine (PC 32:0), phosphatidylethanolamine
(PE 32:0), phosphatidylserine (PS 32:0)
" Separation and identification of phospholipids by hydrophilicinteraction liquid chromatography coupled to tandem high resolutionmass spectrometry with focus on isomeric phosphatidylglycerol andbis(monoacylglycero)phosphate" Christian Vosse¹, Carina Wienken¹, Cristina Cadenas², Heiko Hayen¹, ¹Institute of Inorganic and Analytical Chemistry, University of Münster, Corrensstr. 30, 48149 Münster, Germany ²Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany
Lipids SPE:
Cardiolipin iSPE® HILIC Isolation

Publication outlines a HILIC-based clean‐up method enabling complete separation of polar lipids containing four fatty acyl residues from non-polar lipid classes using iSPE® HILIC columns.
Biometals
![]()
Enterobactin, a catecholate siderophore
Siderophores are small, high-affinity iron-chelating compounds secreted by microorganisms such as bacteria and fungi and serving to transport iron across cell membranes. Siderophores are amongst the strongest soluble Fe3+ binding agents known.
iHILIC® Fusion, 2.1 x 150mm, 3.5µm, 100Å.
Six siderophores of Pseudomonas taiwanensis VLB120 and their
iron(III)- and aluminum(III) complexes were separated
by means of an iHILIC Fusion column. This technique enabled the
separation of the siderophores according to their
different acyl side chain, and additionally, according
to their central ion.
" Mass spectrometric characterization of siderophores produced by Pseudomonas taiwanensis VLB120 assisted by stable isotope labeling of nitrogen source" Karen Scholz¹ Till Tiso² , Lars M. Blank² , Heiko Hayen¹
¹ Institute of Inorganic and Analytical Chemistry, University of Münster, Münster, Germany, ² Institute of Applied Microbiology, RWTH Aachen, Germany
Menu
Enhanced sensitivity of inorganic ion detection with ELSD when using the polymeric Fusion(P):
Elimination of silica leaching increases sensitivity.
![]()
iHILIC® Fusion(P), 4.6 x 150mm, 200Å, 5µm.
Separation of ammonia and sulfate:
Mobile phase: A) Acetonitrile; B) 25 mM ammonium acetate, pH 6.8 (salt solution without pH adjustment)
Gradient program: 0 min: 85% A, 6 min: 65% A, 6-9 min: 65% A, 9-16 min: 85% A.
Flow rate: 0.2 mL/min. Detector: NQAD
Peaks: #2: Ammonium, #3: Sulfate.
iHILIC® Fusion(P), out performs ZIC-pHILIC for analysis of inorganic ions
![]()
iHILIC® Fusion(P), 4.6 x 150mm, 200Å, 5µm.
Gradient: 85% ACN/15% 25 mM ammonium acetate to 60% ACN/40% 25 mM ammonium acetate.
When using iHILIC® Fusion(P), thiosulfate is a sharp symmetrical peak, sulfate elutes after thiosulfate as a sharp symmetrical peak and sodium is more retained than on the ZIC®pHILIC column (shown above).
Data courtesy of Paul Kostel, Eurofins Advantar Labs., Inc.
The Nest Group, Inc.™ 17 Hayward St., Ipswich, MA 01938-2041 USA
Please e-mail to webmaster.
| Home | IdeaBook | Ordering | Price/Applic | Price/Vendor | Protocols|